

Low Cost BioControl AgentsNIPHM has been working on several Biological Agents and low cost insect-pest lures (as indicated below) to promote Bio Intensive strategies for Plant Health Management and Sustainable Agriculture, any Extension worker and Farmer can contact to undergo training on on-farm production of Biological Control Agents. Any Extension officer who can mobilize 30 farmers can be given three days training on on-farm production free of cost for all farmers as well as extension workers. The travel and Food cost can be borne out of state training fund. |
||
I. Biocontrol Agents |
||
Bracon hebetor Say and B. brevicornis (Wesmael) (Hymenoptera: Braconidae) are highly polyphagous, gregarious, and ecto-larval parasitoids of several species of Lepidopteran larvae. They parasitize a variety of important Lepidopteran pests of stored product and field crops (Dabhi et al. 2011). Among the common insect pests that are hosts of Bracon are rice moth (Corcyra cephalonica), angoumois grain moth (Sitotroga cerealella), wax moth (Galleria mellonella), Indian meal moth (Plodia interpunctella), castor shoot and capsule borer (Conogethes punctiferalis), castor semilooper (Achaea janata), cabbage head borer (Hellula undalis), gram pod borer (Helicoverpa armigera, spotted pod borer (Maruca testulalis), spotted bollworm (Earias vittella), tobacco caterpillar (Spodoptera litura), cabbage leaf webber (Crocidolomia binotalis), sorghum/maize stem borer (Chilo partellus), pink bollworm (Pectinophora gossypiella) and coconut black headed caterpillar (Opisina arenosella) (Dabhi et al. 2011, Anonymous 2013). Bracon spp. are important parasitoids of O. arenosella and parasitism was observed throughout the year, it ranged from 26.2 to 26.7% during the peak period of infestation. It attacks the larval stage of the insect host and lays eggs on the surface of the host insect. Larvae upon hatching start feeding on host body fluids by inserting their mouth parts into host. From each host larvae 2 or more parasitoid larvae develop and pupate.

|

|
|

|
| Adult | Larva | Pupae | Egg laying by Bracon |

|

|

|
| Stapling Bracon card with pupae |
Releasing adults collected from sandwich or tub method |
Releasing adults in tub method |

|

|
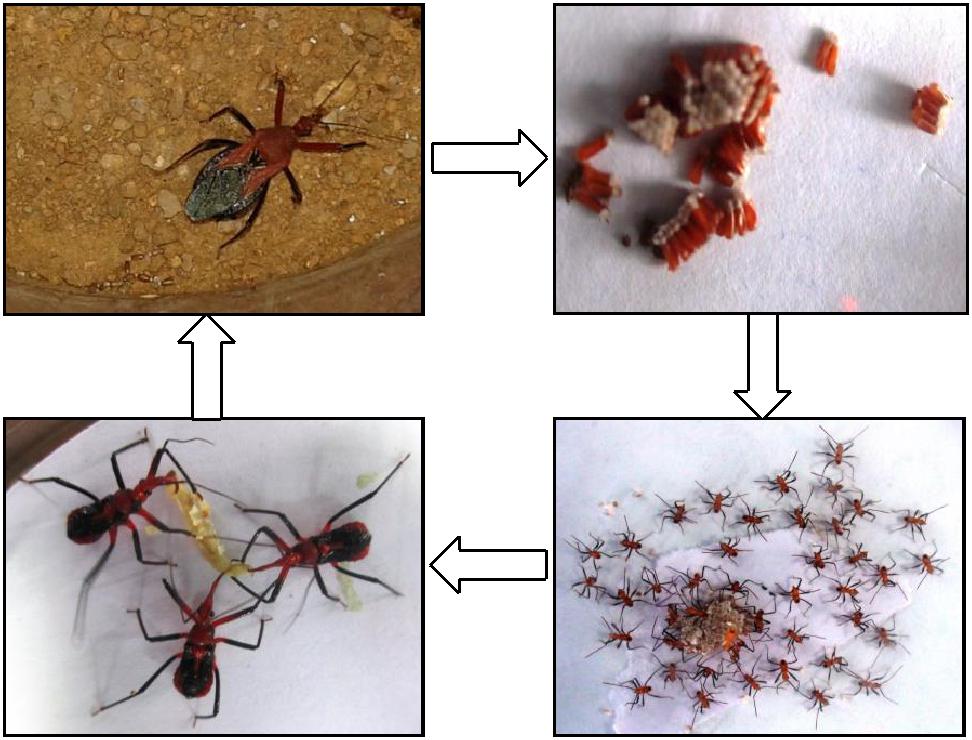
The reduviids, also known as assassin bugs, are predaceous cimicomorphan bugs with cosmopolitan distribution and belong to Reduviidae family. There are about 7,000 species of reduviids reported worldwide. Two reduviid bugs, Rhynocoris marginatus/R. fuscipes, are important and effective predators of insect pests (Ambrose, 2010) in many agro-ecosystems (soybean, groundnut, pigeonpea, cotton, castor, rice, cabbage, tobacco, pumpkin, okra, citrus, sugarcane, sesbania, apple etc.). Predaceous reduviids are of considerable economic importance because they reduce the pest population by killing the host quickly with their highly proteolytic saliva (Maran, 1999). The effectiveness of the reduviids as biocontrol agents has been demonstrated and the field releases usually resulted in quick and effective control of the target pests (Ambrose, 2010).
Adult bugs often range from 4 - 40 mm in length. They commonly have an elongated head with a distinct narrowed neck, long legs, and a prominent, segmented tube for feeding (rostrum/beak). Most species are dark in color with hues of brown, black, red, or orange. Reduviids feed on a wide range of soft-bodied insects mainly caterpillars. The mass rearing of the reduviids is highly successful when offered excess live prey (Edwards, 1962; Ambrose, 2003). Reduviids can be reared on natural or alternative laboratory hosts. In the laboratory, reduviids are mass reared using Corcyra cephalonica larvae as host.
|
Recent trends in agriculture towards reduced pesticide use and ecological sustainability have led to increased interest in spiders as potential biological control agents. For a predator to effectively and economically control an insect pest, the predator must be capable of not only reducing pest densities to levels below an economic threshold, but also to stabilize those pest densities over time. Spiders may be capable of fulfilling both of pest reduction and pest stabilization requirements. Spiders have been successfully used as biocontrol agents in two groups of crop ecosystems throughout the world, orchards (primarily apple) and rice paddies. In rice ecosystem, spiders are the dominant predators controlling several insect pests mainly brown plant hoppter, green leaf hoppers etc.
Conservation of Spiders:The pest management strategy in orchards has been through spider conservation via reduced pesticide use rather than augmentation In rice paddies in Asia, however, spiders are often purposefully introduced into fields. In China, farmers build straw or bamboo shelters for spiders and then move these shelters to whichever paddies are experiencing pest outbreaks. This method of spider augmentation led to a 60% reduction in pesticide use. In Japan, spider populations are maintained and enhanced by the release of Drosophila fruit flies into fields when pest insects are not abundant. Ground dwelling spiders such as lycosids are one of the most important predators of leafhopper and planthopper pests of rice, and the addition of wolf spiders to rice paddies can result in reductions in pest populations similar to that seen with insecticide use. Spiders are more susceptible than insects to most of the insecticides used in agriculture. Spiders can be conserved by 1) providing hedges; 2) spider egg sacs can be collected and covered with hay near the standing crop; 3) placing mulches which provide required humidity and shelter for spiders; and 4) placing straw bundles in rice fields.

|

|

|

|

|

|
The rice meal moth, Corcyra cephalonica Stainton (Lepidoptera: Pyralidae) ranks first in the mass culturing of entomophagous insects due to its amenability to mass production, adaptability to varied rearing conditions and its positive influence on the progeny of the natural enemies.
Corcyra cephalonica (Stainton) a stored grain pest has been proved to be one of the most efficient surrogate host for rearing a wide range of biological control agents. The important among them are egg parasitoids - Trichogramma spp., egg larval parasitoids - Chelonus blackburni, larval parasitoids - Bracon spp., Goniozus nephantidis, Apaneteles angaleti, insect predators - Chrysoperla carnea, Mallanda boniensis. Cyrtorhynus feltiae (Neoaplectana carpocapsea) is reared on the larvae of C. cephalonica. Besides, some entomopathogenic nematodes such as Steinernema feltiae is also reared on the larvae of Corcyra cephalonica . Only an efficient and healthy insect mass rearing medium can result in mass production of effective biological control agents. Corcyra can be mass multiplied throughout the year in all the ecological zones of India at 28± 2°C and 65± 5% Relative Humidity considering the economics as well as quality of eggs produced.
The following materials are required to mass produce Corcyra cephalonica in the laboratory.
| Absorbent cotton | Mosquito net | Storage racks |
| Blotting paper | Moth aspirator (collector) | Streptomycin sulphate |
| Broken sorghum grain | Oviposition drums | Rubber band |
| Camel hair brush | Plastic basin | Measuring cylinder |
| Enamel Tray | Shoe brush | Oven |
| Honey | Soap | Home milling machine |
| Muslin cloth | Specimen tube | Sieves |
| Formaldehyde 40% | Yeast | Moth scale egg separator |
| Filter paper | Filter paper | Face masks |
| Storing drums | Groundnut kernel | Sulphur (WP) |
| Coarse weighing balance |
| * | The host rearing containers (basins) are made of materials which are non-toxic, cheap and optimum sized to permit mating and host searching and amenable to easy cleaning. |
| * | The basins (16" or 18"dia) used for Corcyra multiplication are thoroughly cleaned with 0.5% detergent wash and rinsing in tap water followed by wiping with dry, clean – used towel and shade drying. |
| * | Whenever the trays are emptied after a cycle of rearing, they have to be cleaned preferably to 2% formaldehyde and returned to storage until further use. |
| * | On reuse the cleaning steps are repeated. |

|

|
| * | The requisite quantum of sorghum is milled to make 3-4 pieces of each grain. |
| * | Sorghum grains are heat sterilized in oven at 100°C for 30 minutes and the grains are sprayed with 0.1% formalin. |
| * | This treatment helps in preventing the growth of moulds as well as to increase the grain moisture to the optimum (15-16%), which was lost due to heat sterilization. Then grains are air dried. |

|

|
| * | The primary source of Corcyra eggs is reputed laboratories, commercial producers for bulk preparation. |
| * | If it is intended to begin the production with nucleus colony, the adult moths can be collected from warehouses where the food materials are stored. |
| * | The eggs used for building up the colony of Corcyra have to be free from contaminants like the moth scales and broken limbs and not exposed to UV light. |
| * | The collections of overnight laid eggs are measured volumetrically to ascertain the number of trays that can be infested with eggs. One cc of eggs is known to contain approximately 16000 - 18000 eggs. |
| * | The overall production scheme involves initial infestation of the Sorghum medium with Corcyra eggs in desired quantities. |
| * | This is accomplished by sprinkling the freely flowing eggs on the surface of the medium in individual basins. |
| * | Per basin 0.5 cc eggs of Corcyra are infested. The basins are then covered with clean khadi cloth and held tightly with rubber fasteners. |
| * | Yeast, groundnut kernel and streptomycin is added to enhance egg laying capacity of the adult moths and for enriching the diet. |
| * | The basins are carefully transferred to the racks. |

|
| * | The larvae that hatch out in 3-4 days begin to feed the fortified Sorghum medium. |
| * | At this stage, light webbings are noticed on the surface. As the larvae grow up they move down. |
| * | During this period the larvae are allowed to grow undisturbed in the trays. |
| * | After about 35-40 days of charging, moths start emerging and the emergence continues for two months. |
| * | 10 - 75 moths emerge daily with the peak emergence being between 65th and 75th day. |
| * | Adults are either aspirated with mechanical moth collector or collected with specimen tubes. The whole operation is carried out in a tent of mosquito net. |
| * | This prevents the large-scale escape of the moths, which if uncontrolled can migrate to the storage area and spoil the grains stored by laying eggs. |
| * | Workers involved in the collection of moths should wear face moths continuously to avoid inhalation of scales. |
| * | Collect the moths daily and transfer to the specially designed oviposition cages. |
| * | The adults are provided feed containing honey solution. The adult feed is prepared by mixing 50 ml honey with 50 ml water and 5 capsules of vitamin E (Evion). |
| * | The feed is stored in refrigerator and used as and when required. Piece of cotton wool tied with a thread is soaked in the solution and inserted into the drum through the slot at the top. |
| * | From a basin, moths can be collected up to 90 days after which the number of moths emerging dwindles down and keeping the basins is not economical for the producer. |
| * | The moths lay the eggs in large numbers loosely. The scales and broken limbs are also found in larger quantities along with the eggs. |
| * | They cause potential hazard to the workers after years of working in Corcyra laboratory. To minimize the risk of scales freely floating in the air, the oviposition drums are placed on sheets of filter paper in enamel trays which trap effectively the scales. |
| * | Sets of several oviposition cages are kept in ventilated place near an exhaust fan to enable the workers comfort. |
| * | Daily morning the oviposition cages are lifted up and the wire-mesh bottoms are cleaned gently with a shoe brush so that the eggs and remnants of scales and limbs settled on the mesh are collected along with those on the filter paper. |
| * | The collections are cleaned by gently rolling the eggs on filter paper to another container. Then they are passed to sieves in series and finally clean eggs are collected. |
| * | The eggs are quantified in measuring cylinders and used for building up the stocks and natural enemy production. |

|

|
| Rearing of Corcyra in plastic basins | |
| Accurate information is needed on the history of individual basins. The following information is furnished. | |
| I | Date of egg infestation |
| II | Date of preparation of feed |
| III | Source of Egg |
| IV | Expected date of adult emergence |
| V | Daily collection of moths |
| VI | Problems encountered with the basin during production |
| VII | Personnel handling the basin |
| * | Bacterial disease some time attacks Corcyra culture. To control this, streptomycin sulphate is added to the crushed sorghum @0.23 kg-1 and mixed thoroughly. |
| * | Occasionally the mite Pyemotes ventricosus (Newport) contaminate the culture and affect egg laying and larval development of Corcyra. |
| * | In case of infestation the racks, cages, boxes etc. should be disinfected with formalin and placed in the sun for six hours. |
| * | Boxes containing developing larvae should be dusted with sulphur so that a thin layer of sulphur is present over the sorghum. |
| * | In case of severe infestation acaricide dicofol (Kelthane) can be applied. For this muslin sheets are dipped in a 0.05% solution of dicofol and air dried for couple of hours. |
| * | The sheets are spread over the sorghum grains in boxes. Mites coming in contact with the treated cloth are killed rapidly. |
| * | Control of Bracon hebetor infestation: A plastic tub filled with water near racks containing Corcyra boxes and keep a table lamp with 60 or 100 W bulb. The light of the table lamp should face the surface of water. Parasitoids will be attracted towards light and in turn will fall into the water and die. |
| * | Another method to trap them is a fine film of some sticky material on the glass window pane and directing the light of table lamp towards glass pane. |
| * | The trap should be operated daily. Side by side the culture should also be replaced with fresh stock. |
| * | The boxes should be sterilized by spraying 1% formaldehyde or sodium hypochlorite solution and sun dried for 6 hours. Care should be taken to remove host/parasitoid cocoons from boxes. |
| * | The feed material jowar should also be streilized in the same manner as mentioned above. Mix all ingredients in separate room and charge with Corcyra eggs. Close the lid and these sterilized boxes should not be open for 40 days and kept in a separate room. By this method Bracon can be eliminated from the culture. |
| * | In case of labs having separate Corcyra rearing rooms on one corner disinfect all boxes one by one and keep in one room. All the Bracons in previous room can be killed by spraying low concentration of any pesticide. All the windows and doors can be kept open for 15 days. |
| * | Thereafter sprinkle whole room with 1% formaldehyde (40%) solution. After about 10 days bring back whole culture to same room. Thereafter regularly keep light-water-trap to monitor Bracon. |
| * | Discoloured larvae, white cocoons if found are collected and immersed in 0.5% formaldehyde to kill the parasitoids. |
| * | Provide windows with fine iron mesh and wire mesh double door to avoid any entry of parasitoids. |
A parthenogenetic egg-larval parasitoid, Chelonus blackburnii has a fairly wide host range but in India the common meal moth Corcyra cephalonica and potato tuber moth (PTM) Phthorimaea operculella have often been used for multiplication of this parasitoid. C. blackburnii is introduced from Hawaii. It could also be multiplied successfully on Spodoptera exigua. C. blackburnii has been used for the biological suppression of P. operculella, Earias vitella, Pectinophora gossypiella and Helicoverpa armigera on cotton and other host plants in many states. It is becoming an important component of IPM systems on potato, cotton, etc.,
Mass Production procedure :| * | Take 100 fresh corcyra eggs of 0-24 hr old which are not exposed to UV and paste to 5 x 5 cm card. This card containing eggs is exposed to 30 adults of C. blackburnii adults in a 1.5 L container for parasitisation. |
| * | The plastic container has windows with plastic mesh for aeration. Two cotton swabs, one soaked in 10% honey solution and the other in drinking water are also placed inside from the side opening which is closed tightly with a cloth covered cotton plug. |
| * | The egg card after exposing to C. blackburnii for 24 hr is removed and placed on 500 g sterilized cumbu medium. In 30 days time, adults start emerging from the cocoons formed in the cumbu (pearl millet) medium after completing development on Corcyra larvae. The adults live for 25 days and their fecundity is about 400 eggs. |
| * | The parasitoid could also be reared on potato tuber moth (PTM). A set of 1500 eggs are laid on a cloth are stapled to a card. |
| * | The plastic container (14 cm x 11 cm) with parasitised eggs is converted into C. blackburnii rearing unit by cutting windows and fixing plastic mesh for aeration. |
| * | Two cotton swabs, one soaked in 50% honey solution and the other in drinking water are also placed inside from the side opening which is closed tightly with a cloth covered cotton plug. |
| * | The PTM egg card after exposing to C. blackburnii for 24 hr is removed and placed on punctured potatoes. This provides more entry points for PTM larvae and kept in a similar plastic container as described for exposure to C. blackburnii. |
| * | The bottom of this container is lined with sterilized sand. In 25-27 days time, adults start emerging from the cocoons formed in sand at the bottom of the cage or sometimes inside potatoes after completing development on potatoes. |
| * | The adults live for 23-31 days and their fecundity is about 288-390. Parasitoid host ratio of 1:50 should be maintained and the fresh lot of eggs provided every day. |

|

|
| Rearing of Chelonus blackburnii | |
| * | Potato tuber moth: Two releases @50,000 adults release-1 in the field and 5 adults kg-1 potatoes in godowns |
| * | Cotton bollworms: 50,000 adults week-1, first release coinciding with sighting of eggs in the field |
| * | Helicoverpa armigera at weekly intervals, first release coinciding with sighting of eggs in the field |
The genus Trichogramma is cosmopolitan in distribution and present in all terrestrial habitats and is one of 80 genera in the family Trichogrammatidae. Trichogramma are primary parasitoids eggs of Lepidoptera, but parasitism also occurs in eggs of other orders such as Coleoptera, Diptera, Hemiptera, Hymenoptera and Neuroptera. It is important for plant protection because of its wide spread natural occurrence and its success as biological control agent by mass releasing. Since this parasitoid kills the pest in the egg stage itself before the pest could cause any damage to the crop and also that it is quite amenable to mass production in the laboratories, it has the distinction of being the highest produced and most utilized biological control agent in the world Trichogrammatidae includes the smallest of insects, ranging in size from 0.2 - 1.5 mm.
Biology of Trichogramma :The development of all Trichogramma spp. is very similar. Being an egg parasite, the female drills a hole through the chorion and deposits its eggs within the egg of the host. The internal pressure of the egg forces a small drop of yolk out of the oviposition hole. Females feed on this yolk, which increases their longevity and under laboratory conditions a female parasitizes from 1-10 eggs day-1 or from 10-190 during her life. Large females parasitize more eggs than smaller females. The number of eggs laid per host egg may vary from 1-20 or more depending upon the size of the host egg. However in sugarcane, in which moth borer eggs are small, generally 1 or 2 parasites develop egg.-1
A female parasitoid can distinguish already parasitised eggs, thereby avoiding superparasitism or multiple-parasitism under natural conditions. Fecundity varies from 20 - 200 eggs female-1 according to the species, the host, and the longevity of the adult. Eggs in the early stages of development are more suitable for parasite development. Older eggs, especially those in which the head capsule of the larva is visible, are not usually parasitized and if they are, parasite survival is much lower. Venom injected by the female at the time of oviposition is believed to cause this pre-digestion of the eggs contents.
During the 3rd instar (3 - 4 days after the host egg was parasitized) dark melanin granules are deposited on the inner surface of the egg chorion, causing the host egg to turn black. This is an invaluable diagnostic character for distinguishing them from unparasitised eggs. Larvae then transform to the inactive pupal stage.
The adult wasps emerge from the pupae and escape the host egg by chewing a circular hole in the egg shell. The black layer inside the chorion and the exit hole are evidence of parasitism by Trichogramma. The egg, larval and pupal stages of Trichogramma at 28± 20°C are completed in about 1 day, 3 - 4 days, and 4 - 5 days respectively. Thus, the life cycle is completed in 8 - 10 days, but it may be prolonged at lower temperatures or hampered at very high temperatures. The adults are short lived (6-8 days). Mating and oviposition take place immediately after emergence. The sex ratio is generally 1:1.
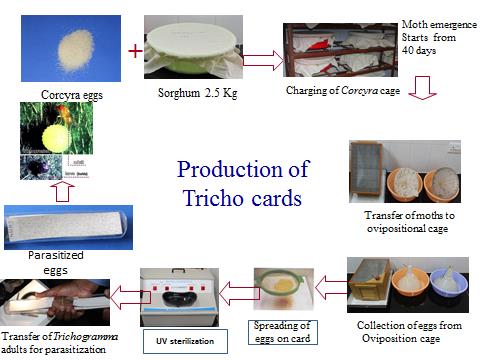
Preparation of Tricho cards :| * | The parasitisation of Trichogramma spp., in laboratory condition on one cc eggs of Corcyra cephalonica, which are uniformly spread and pasted on a card measuring 15 cm x 10 cm is called as Tricho card. The card has 12 demarcations (stamps). |
| * | About 12,000 Trichogramma adults emerge out from this card in 7-8 days after parasitisation. |
| * | To delay the emergence of Trichogramma, these cards can be stored in refrigerator at 5-10°C for 10-15 days. |
| * | On removing the cards to room temperature, the parasitoids emerge normally. Tricho cards have a shelf life of 2-3 days. However, these can be stored in a refrigerator for a period of 1 month without any spoilage. |
| * | Label information on the manufacturer, species of the parasitoid, date of parasitization and expected date of emergence are given in the left over spaces. |
| * | A coat of 10% gum arabic is applied on the grids and the eggs are sprinkled uniformly in a single layer with the aid of a tea strainer. |
| * | The excess eggs pasted are removed by gently passing a shoe brush over the card after sufficient air drying under fan. |
| * | The egg cards are placed into polythene bags of suitable size and the nucleus card of Trichogramma are introduced in it. |
| * | The easiest way to accomplish this is to place a piece of Tricho egg card containing parasitized eggs (i.e. pharate adults) that are ready to yield the adults and to hold them in subdued light for 2 - 3 days. |
| * | The emerging parasites readily parasitize the fresh eggs. |
Poor quality of mass reared Trichogramma can result in control failures. The artificial conditions of mass rearing can select for genetic changes that reduce the effectiveness of the Trichogramma in the field. Such rearing conditions include rearing multiple generations on unnatural host eggs, the absence of plants, crowding and interference, rapid generation time, and failure to rejuvenate genetic stock. Except for obvious problems such as lack of adult emergence or wing deformities, growers and pest consultants cannot detect poor quality Trichogramma prior to release. Commercial suppliers are responsible for maintaining desirable characteristics necessary for good performance in the field. Production colonies should be periodically replaced with individuals from a stock culture maintained on the natural or target host. Suppliers also should assess the per cent host egg parasitization, adult emergence, and the sex ratio of emerged adults to be sure they are within acceptable standards. Standards for established cultures on Corcyra are 95.5% egg parasitization, 90.5% adult emergence and a sex ratio of 1 : 1.5 females male-1.
| * | Tricho cards should be packed in such a way that the parasitised surface is on the inner side. |
| * | Emergence date should be specified on cards for the guidance of the users. |
| * | Tricho cards should be stapled on the inner-side of the leaf to avoid direct sunlight. |
| * | Card should be stapled in morning hours and just before emergence to avoid predation. |
| * | Farmers should refrain from using pesticides in the field where Trichogramma are released. If need arises selective / safer pesticides can be used and it is to be ensured that pesticides are used 15 days before or after release of Trichogramma. |
| T.chilonis (different strains for different crops) | Sugarcane borers |
| Tomato fruit borer | |
| Cotton bollworm | |
| Maize stem borer | |
| Citrus leaf eating caterpillar | |
| T.exiguum | Maize stem borer |
| T. achaeae | Cotton spotted bollworm |
| T. japonicum | Sugarcane top borer |
| Paddy stem borer | |
| T.brasiliensis | Cotton bollworm |
| Tomato fruit borer | |
| T. embryophagum | Apple Codling moth |